- Written By
Shreya_S
- Last Modified 15-02-2025
Osmoregulation: Definition, Mechanisms & Importance in Biology
Osmoregulation: Organisms require osmoregulation to maintain a constant and proper osmotic pressure within the body or cell. Water potential regulation within a cell or organism maintains fluid and electrolyte balance in proportion to the surrounding environment. As an example, organisms use excretion (such as getting rid of metabolic wastes and other substances toxic to the body when they are in large amounts).
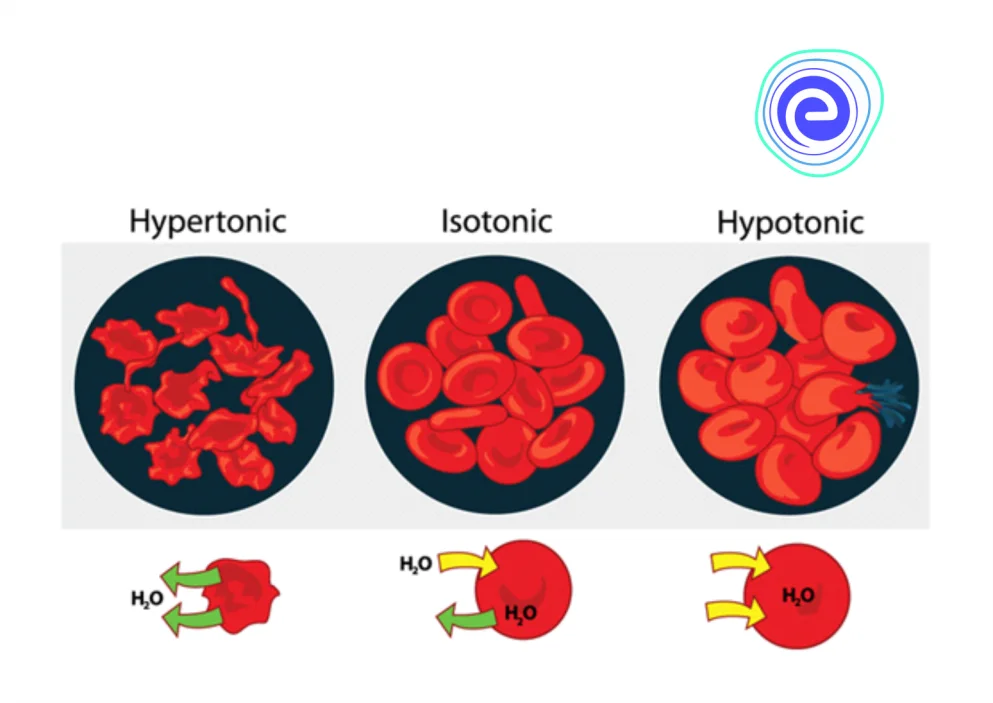
In a hypertonic environment, cells shrivel as a result of the loss of water. In a hypotonic environment, water intake causes cells to expand. By maintaining an isotonic environment, the blood prevents cells from shrinking or expanding. Let’s learn about Osmoregulation, definition, mechanism, types, and significance.
Definition of Osmoregulation
The process of maintaining salt and water balance (osmotic equilibrium) across membranes in the body is known as osmoregulation. Water, electrolytes, and non-electrolytes make up the fluids inside and around cells. An electrolyte is a substance that, when dissolved in water, separates into ions. In water, a non-electrolyte, on the other hand, does not break down into ions.
Blood plasma, fluid within cells, and interstitial fluid, which exists between cells and tissues of the body, are all examples of bodily fluids. Semipermeable membranes make up the body’s membranes (the membranes that surround cells and the “membranes” made up of cells that line internal cavities). Semipermeable membranes allow certain types of solutes and water to pass through, although cell membranes are generally impermeable to solutes.
- Despite external stimuli such as temperature, nutrition, and meteorological conditions, osmoregulation maintains the right balance of electrolytes in the human body.
- Osmotic balance ensures that ideal concentrations of electrolytes and non-electrolytes are maintained in cells, bodily tissues, and interstitial fluid by the diffusion of water or solutes.
- Solutes or water passes over a semipermeable membrane, causing concentrations on both sides to equalise.
- Hypotonic solutions cause cells to inflate as water passes through the membrane, whereas hypertonic solutions cause cells to shrivel as water passes through the membrane.
- Water flow across membranes caused by osmotic pressure can alter the volume of the body’s fluid compartments, affecting medical indications such as blood pressure.
Fig: Red Blood Cells’ Responses to Hypertonic, Hypotonic, and Isotonic Solutions
Mechanism of Osmoregulation
- Animals tend to maintain an osmotic concentration that is optimal for their habitat. They have developed a variety of adaptations to maintain a consistent internal osmotic concentration, as follows:
a. To limit the area of permeability, an impermeable covering (such as an exoskeleton or cuticle) is developed over the body surface.
b. The permeability of the exposed regions of the body is reduced.
c. To compensate for their loss, the absorption of water and salts through excretory organs, the gut wall, or the body wall is increased.
d. To save water, wastes are removed in semisolid form.
- The reduced permeability of the body surface and the development of kidneys play a large influence in this occurrence. Hence the osmoregulatory mechanisms in vertebrates are more efficient than invertebrates.
- The body fluids are more concentrated than the surrounding water; therefore all freshwater species are hypertonic to it. As a result, there is a tendency for water to infiltrate their bodies and salt to diffuse outward via their semipermeable body walls. If this process continues without a regulating mechanism, the body’s essential salts will be lost, resulting in blood dilution. The kidneys of freshwater animals excrete hypotonic urine, which is dilute urine with a lot of water and very few solutes. Water conservation is not an issue for such animals.
- The surrounding air in the terrestrial habitat, on the other hand, is devoid of both water and salts. As a result, terrestrial animals frequently experience water and salt loss. Sweat and urine can cause water loss. Water loss can occur as a result of evaporation from the skin’s surface, perspiration, or urine. Sweat or urine can cause salt loss. The only method to solve this problem is to maintain a balance between water and salt loss and uptake. To compensate for the lack of water and salts, such animals drink a lot of water and eat a lot of salty food. Their kidneys excrete hypertonic urine, which has a higher concentration of solutes than blood plasma.
- The urine is hypertonic in the summer when there is a lot of perspiration and hypotonic in the winter when there isn’t a lot of sweating. The volume and composition of urine differ depending on the body’s physiological state. Despite large variations in water and salt intake, the kidneys keep the body fluid composition moving by controlling the amount of nitrogen wastes, salts, fluid, and ionic concentration in the blood.
Types of Osmoregulation
There are two major types of osmoregulation:
1. Osmoconformers: Osmoconformers are organisms that try to adapt their body’s osmolarity to that of their environment. In other words, inside the body, these organisms retain the same osmotic pressure as outside water. They conform in one of two ways: actively or passively. Most marine creatures may be isotonic with seawater (osmoconformers). The concentrations of their bodily fluids adjust to changes in seawater concentration.
2. Osmoregulators: Osmoregulators are organisms that actively control their osmotic pressure without relying on their surroundings. Humans, like many other vertebrates, are osmoregulatory. The majority of freshwater fish are also osmoregulatory.
Osmoregulation in Different Organisms
Different organisms exhibit different types of osmoregulation. Following are some osmoregulation processes in different organisms:
- Osmoregulation in Freshwater Fish
Goldfish and other stenohaline species can only tolerate a small range of salinity. Only about 90% of bony fish species can live in both freshwater and the ocean. In the alternate habitat, these fish are unable to regulate their osmotic pressure.
- Osmoregulation in Marine Fish
Shark blood includes urea and trimethylamine oxide (TMAO). The electrolyte content of the shark’s blood differs from that of seawater, yet it maintains isotonicity with seawater by storing urea in high amounts. Sharks are “ureotelic” animals, meaning they excrete urea to keep their osmotic balance in check. TMAO stabilises proteins in the presence of high urea levels, avoiding peptide bond breakage that would otherwise occur at such high urea levels.
- Osmoregulation of Euryhaline
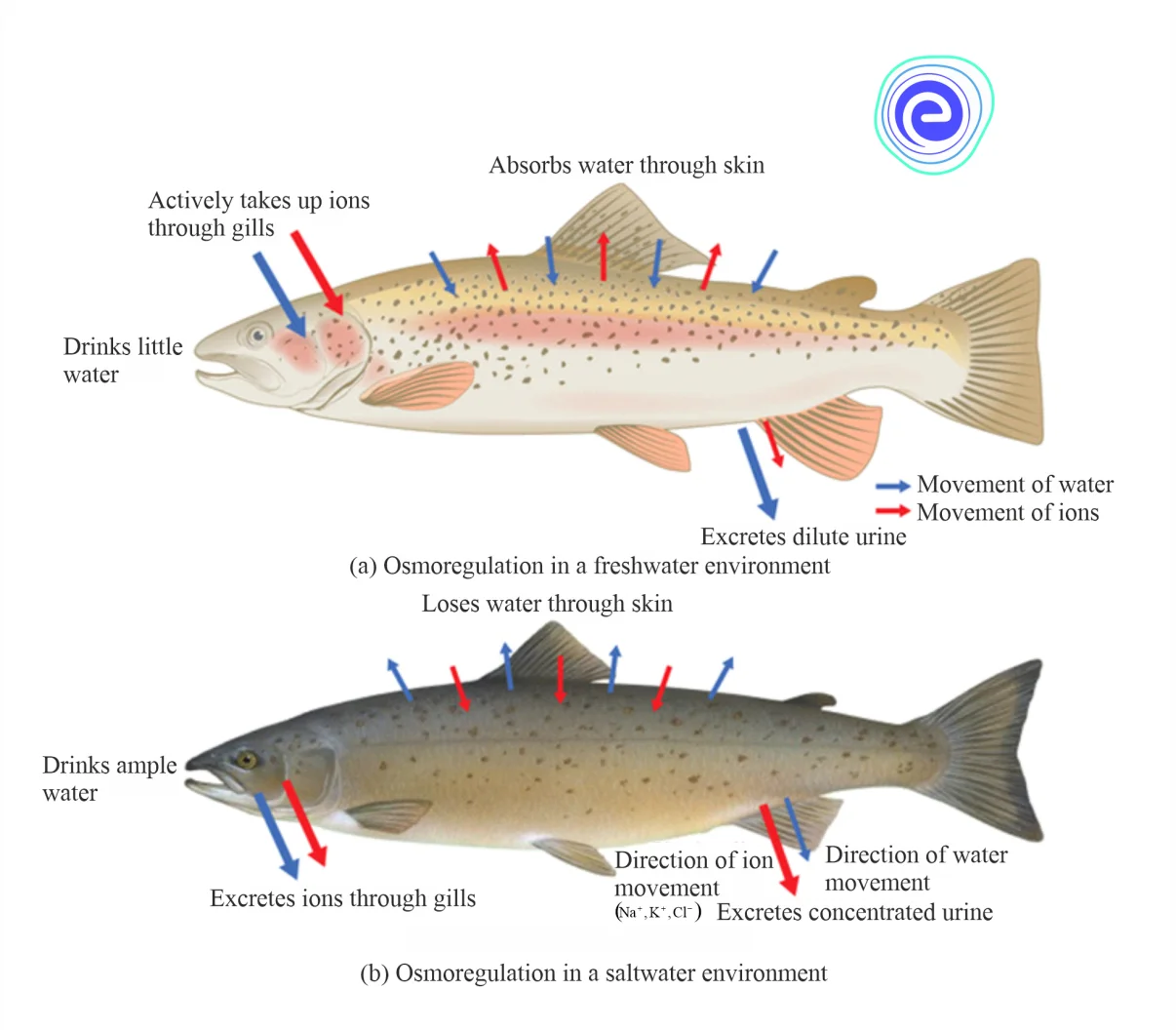
A few species known as euryhaline creatures, on the other hand, spend part of their life cycle in freshwater and the rest in seawater. The species, such as salmon, can tolerate a wide variety of salt levels. To thrive in a range of aquatic habitats, they developed osmoregulatory mechanisms. Their skin absorbs water in freshwater that is relatively hypotonic (low osmotic pressure). The fish do not drink much water and maintain electrolyte balance by avoiding dilute urine and vigorously absorbing salts through their gills. The salmon lose water when they relocate to a hypertonic saltwater habitat, excreting the extra salts through their gills and urine.
Fig: Osmoregulation in (a) Freshwater (b) Saltwater Environments
Osmoregulation in Humans
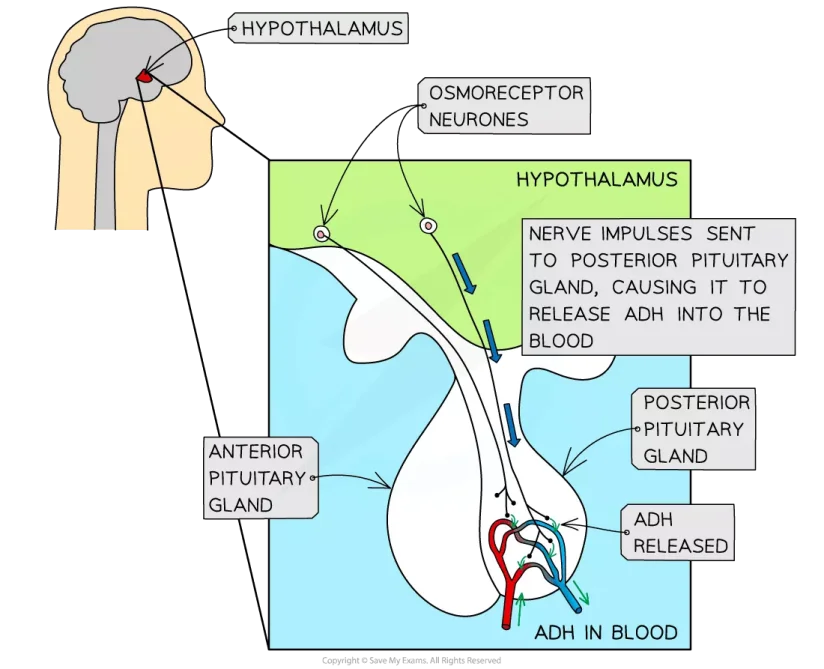
Fig: Osmoregulation in Humans
In humans, the kidney is the primary organ for osmoregulation. The kidneys reabsorb water, amino acids, and glucose. When the body’s water level is too high, it produces a lot of hypotonic urine. It holds water and generates a small volume of hypertonic urine when the water level is low. As a result, the kidneys keep the body’s electrolytic balance in check. The absorption process is regulated by aldosterone, angiotensin II, and antidiuretic hormones. Perspiration also loses some water and electrolytes.
The thirst and secretion of ADH are controlled by osmoreceptors in the hypothalamus of the brain. Aquaporins’ water channels are opened by ADH, allowing water to flow. As a result, the kidneys continue to absorb water until the pituitary gland stops producing ADH.
Effect of ADH on the Kidneys
- Osmosis reabsorbs water from the filtrate in the nephron.
- As the filtrate flows through structures known as collecting ducts, ADH induces the luminal membranes of the collecting duct cells (those facing the lumen of the nephron) to become more permeable to water.
- ADH accomplishes this by increasing the number of aquaporins (water-permeable channels) in the collecting duct cells’ luminal membranes. This takes place in the following manner:
a. The membranes of collecting duct cells contain multiple aquaporins, and ADH molecules attach to receptor proteins, starting a signalling cascade that leads to the phosphorylation of the aquaporin molecules.
b. The aquaporins are activated, forcing the vesicles to fuse with the collecting duct cells’ luminal membranes.
c. This enhances the membrane’s Swater permeability.
- Water molecules flow from the collecting duct (high water potential) through the aquaporins and into the tissue fluid and blood plasma in the medulla as the filtrate in the nephron travels through the collecting duct (low water potential).
- The filtrate in the collecting duct becomes more concentrated as it loses water.
- A small amount of concentrated urine is produced as a result. This travels from the kidneys to the bladder via the ureters.
Significance of Osmoregulation
By eating food and water and secreting sweat, excreting urine, and faeces, complex multicellular animals exchange water and nutrients with the environment. When disease or injury disrupts the processes that control osmotic pressure, toxic waste or water can accumulate, posing a serious health risk.
Mammalian systems have evolved to control osmotic pressure by balancing electrolyte concentrations in three primary fluids: blood plasma, extracellular fluid, and intracellular fluid. Water migration across membranes due to osmotic pressure can alter the volume of these fluid compartments. Osmotic pressure can directly affect blood pressure because blood plasma is one of the fluid components.
Summary of Osmoregulation
The osmotic pressure of fluids and the electrolytic balance in organisms are both regulated by osmoregulation. Osmoreceptors, which detect changes in osmotic pressure, are responsible for this process in mammals. The hypothalamus contains osmoreceptors, which are found in humans and most other warm-blooded animals. Osmoregulators are also found in the kidneys, in addition to the brain. In many marine organisms, osmosis (the passage of solvent through a semipermeable membrane) occurs without any need for regulatory mechanisms because the cells have the same osmotic pressure as the sea. Other organisms, however, must actively take on, conserve, or excrete water or salts in order to maintain their internal water-mineral content.
Frequently Asked Questions (FAQs)
Q.1. What is Osmoregulation, and why is it essential in living organisms?
Ans: Osmoregulation refers to the physiological processes that maintain a fixed concentration of cell membrane-impermeable molecules and ions in the fluid that surrounds cells.
Q.2. Why does osmoregulation occur?
Ans: Osmoregulation maintains the proper balance of electrolytes in the human body, despite external factors such as temperature, diet, and weather conditions.
Q.3. What is the importance of osmoregulation?
Ans: Osmoregulation is an essential process in both plants and animals. It allows organisms to maintain a balance between water and minerals at the cellular level despite changes in the external environment.
Q.4. Which hormone is responsible for osmoregulation?
Ans: Antidiuretic hormone (ADH) has the primary role in osmoregulation by controlling the amount of urine formation. The body maintains water and electrolytes concentration at a relatively constant level by the mechanism of osmoregulation.
Q.5. What happens if there is no osmoregulation?
Ans: Without a mechanism to regulate osmotic pressure, or when a disease damages this mechanism, there is a tendency to accumulate toxic waste and water, which can have dire consequences.
Learn About Antigens and Antibodies Here
We hope this detailed article on Osmoregulation is helpful to you. If you have any queries, drop a comment below, and we will get back to you.