- Written By
Nithya Samanta
- Last Modified 18-03-2025
Qualitative Analysis of Organic Compounds
Qualitative analysis can be defined as ‘unquantified’ or non-quantifiable data in any given compound. In other words, the qualitative analysis provides a detailed analysis of the kind of species present in an organic compound. So, unlike quantitative analysis, which analyses the amount of each ion or element present, qualitative analysis only looks at the kind or nature of elements or chemical entities present in a compound under examination.
Qualitative analysis of organic compounds is carried out to determine their structure and characterisation after they are prepared in their pure form. The analysis gives a perception of the elements present in the compound. Since the most common elements found in the organic compounds are carbon, hydrogen, oxygen, and nitrogen (in some), they are detected through the analysis. Additional elements such as sulphur, halogens, metals, and phosphorus are occasionally present and are detected through qualitative analysis.
Detection of Carbon and Hydrogen
Principle
The carbon and hydrogen present in the organic compound can be detected using dry copper (II) oxide or cupric oxide. The organic compound is heated with dry copper (II) oxide in a hard glass tube. The carbon present in the compound will be oxidised to carbon dioxide, and the hydrogen in it will get oxidised to water. The reactions are as follows:
\(\mathrm{C}+2 \mathrm{CuO} \Delta \rightarrow \mathrm{CO}_{2}+2 \mathrm{Cu}\)
\(2 \mathrm{H}+2 \mathrm{CuO} \Delta \rightarrow \mathrm{HO}_{2}+2 \mathrm{Cu}\)
As an example, if the organic compound under test consists of a molecular formula \({{\rm{C}}_{\rm{x}}}{{\rm{H}}_{\rm{y}}}\) of then the complete combustion of the compound in the presence of cupric oxide can be written as:
\({{\rm{C}}_{\rm{x}}}{{\rm{H}}_{\rm{y}}}{\rm{ + }}\left( {{\rm{2x + }}\frac{{\rm{y}}}{{\rm{2}}}} \right){\rm{CuO\Delta }} \to {\rm{xC}}{{\rm{O}}_{\rm{2}}}{\rm{ + }}\frac{{\rm{y}}}{{\rm{2}}}{{\rm{H}}_{\rm{2}}}{\rm{O + }}\left( {{\rm{2x + }}\frac{{\rm{y}}}{{\rm{2}}}} \right){\rm{Cu}}\)
Carbon dioxide can be detected by its ability to turn lime water milky (Calcium Carbonate is formed). Water can be detected by its ability to get condensed on the cool parts of the test tube. It also turns anhydrous copper sulphate ‘blue’.
\({\rm{Ca}}{({\rm{OH}})_2} + {\rm{C}}{{\rm{O}}_2} \to {\rm{CaC}}{{\rm{O}}_3} + {{\rm{H}}_2}{\rm{O}}\)
\({\rm{CuS}}{{\rm{O}}_4} + 5{{\rm{H}}_2}{\rm{O}} \to {\rm{CuS}}{{\rm{O}}_4} \cdot 5{{\rm{H}}_2}{\rm{O}}\)
Procedure
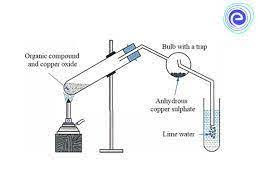
In the laboratory, a small quantity of the dry and pure organic compound is taken and is mixed with about \(5-6\) times of dry and pure cupric oxide powder by weight. The mixture is then strongly heated in a hard test tube which is fitted with a delivery tube. The delivery tube consists of a bulb in the centre, as shown, and is dipped into the lime water in the other end.
The bulb of the delivery tube is packed with glass wool and anhydrous copper sulphate. When heated, carbon gets oxidized to carbon dioxide and turns the lime water milky. Hydrogen in the organic compound gets oxidized to water and turns the anhydrous copper sulphate on the bulb to blue, indicating its presence.
Detection of Nitrogen
Some organic compounds, like amines and nitro compounds, contain nitrogen. The detection of nitrogen in these organic compounds is done using a series of tests as follows:
a. Dry heating test: When the organic compound under investigation is strongly heated, and if it emits a burnt hair or feather smell, it confirms the presence of nitrogen. There are, however, limitations to the test since many compounds containing nitrogen do not emit the smell.
b. Soda-lime test: A small amount of organic compound is heated strongly with soda lime \((\mathrm{NaOH}+\mathrm{CaO})\) in a dry test tube. If the fumes smell of ammonia, it indicates the presence of nitrogen.
\(\mathrm{NH}_{2} \mathrm{CONH}_{2}+2 \mathrm{NaOH} \mathrm{CaO}, \Delta \rightarrow 2 \mathrm{NH}_{3}+\mathrm{Na}_{2} \mathrm{CO}_{3}\)
However, there are limitations to these tests, since many organic compounds containing Nitro and Azo \({\rm{( – N = N – )}}\) groups do not show results to this test.
c. Lassaigne’s test: Lassaigne’s test is used to detect nitrogen, halogens, and sulphur in an organic compound. This is one of the most reliable tests used to detect these components. The elements present in the organic compounds are fused with sodium metal to convert them from covalent to their ionic form.
i) Preparing Lassaigne’s extract
A small piece of sodium is taken and heated strongly until it becomes a globule in a fusion tube. Once the globule is formed, the tube is removed from the flame, and a small amount of organic compound is added to the tube and heated again strongly till the tube becomes red hot. Meanwhile, a China dish with \(10-15\) ml of water is kept ready. When the tube becomes red hot, it is plunged into the China dish containing water and is filtered. The filtrate obtained is called the Sodium fusion extract or the Lassaigne’s Extract.
ii) Testing for Nitrogen element
The Sodium fusion extract, thus prepared, is alkaline since the sodium present reacts with the excess water to form sodium hydroxide. Sometimes, sodium hydroxide is added to ensure the solution is alkaline. A freshly prepared ferrous sulphate \((\mathrm{FeSO}_4)\) is added to this alkaline solution of Sodium fusion extract. The mixture is then warmed a little and cooled. A little dilute sulphuric acid is added to acidify the solution. If nitrogen is present, the solution will turn green or blue in colour. If the solution turns a blood-red colour, it indicates the presence of nitrogen and sulphur in the organic compound.
The reactions for the above test are as follows:
The carbon and nitrogen present in the organic compound forms sodium cyanide during the fusion process, as shown:
\(\mathrm{Na}+\mathrm{C}+\mathrm{N} \rightarrow \mathrm{NaCN}\)
When heated with ferrous sulphate solution, sodium ferrocyanide or sodium hexacyanoferrate (II) is formed and some of the \(\mathrm{Fe}^{2+}\) or ferrous ions are oxidised to ferric \(\left(\mathrm{Fe}^{3+}\right)\) ions. The ferric ions react with sodium hexacyanoferrate (II) to form Iron (II) hexacyanoferrate (II) or ferriferrocyanide, to give a Prussian blue colour.
\(2 \mathrm{NaCN}+\mathrm{FeSO}_{4} \rightarrow \mathrm{Na}_{2} \mathrm{SO}_{4}+\mathrm{Fe}(\mathrm{CN})_{2}\)
\({\rm{Fe}}{({\rm{CN}})_2} + 4{\rm{NaCN}} \to {\rm{N}}{{\rm{a}}_4}\left[ {{\rm{Fe}}{{({\rm{CN}})}_6}} \right]\) Sodiumhexacyanoferrate (ii)
\(3 \mathrm{Na}_{4}\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]+4 \mathrm{Fe}^{3+} \rightarrow \mathrm{Fe}_{4}\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]_{3}\)
Detection of Halogens
The halogens, if present in the organic compound, can be detected using the following tests:
a. Beilstein’s Test: Beilstein’s test is a simple, sensitive, and effective test for detecting halogens in organic compounds. Here, a clean and thick copper wire is heated in the non-luminous flame from the Bunsen burner until the flame’s bluish-green or greenish colour ceases to exist. The wire is then dipped into the organic compound and again shown to the flame. If the flame turns bluish-green or green, it indicates the formation of cupric halides, and therefore, the presence of halogen in the compound. The test has some limitations because even compounds like urea and thiourea, etc., show this test due to the formation of volatile cupric cyanide. Also, it does not indicate the type of halogens (bromine, chlorine, or iodine) present in the compound.
b. Lassaigne’s Test: It is a very reliable test for detecting the presence of halogens in an organic compound. The first step involves the preparation of Sodium Fusion Extract or the Lassaigne’s Extract. The halogens present in the organic compounds are converted to sodium halides:
\(\mathrm{Na}+\mathrm{X} \rightarrow \mathrm{NaX}\)
The next step involves boiling a part of the extract with dilute nitric acid and colling the solution. To this, a few drops of silver nitrate solution is added and tested for below precipitates:
- If a white precipitate is formed, which is soluble in ammonia and insoluble in dil. \(\mathrm{HNO}_{3}\), it indicates chlorine.
- If a pale-yellow precipitate is formed which is partially soluble in ammonia, it indicates the presence of bromine.
- If a yellow precipitate is formed which is insoluble in ammonia, then it indicates the presence of iodine.
Detection of Sulphur
Sulphur, present in an organic compound can be detected using the following tests:
i) Lassaigne’s Test: If an organic compound containing sulphur is fused with sodium, the extract prepared will contain sodium sulphide.
\({\rm{2Na + S}} \to {\rm{N}}{{\rm{a}}_{\rm{2}}}{\rm{S}}\)
The following tests will confirm the presence of sulphur in the compound:
ii) Sodium Nitroprusside Test: When a small portion of sodium fusion extract is treated with a few drops of sodium nitroprusside, it will give a violet colour if the compound contains sulphur. The violet colour will fade slowly on standing.
\(\mathrm{Na}_{2} \mathrm{~S}+\mathrm{Na}_{4}\left[\mathrm{Fe}(\mathrm{CN})_{5}(\mathrm{NO})\right] \rightarrow \mathrm{Na}_{4}\left[\mathrm{Fe}(\mathrm{CN})_{5}(\mathrm{NOS})\right]\)
iii) Lead Acetate Test: In the next half of the portion, dilute acetic acid is added to acidify it and a few drops of lead acetate is added to it. If a black precipitate indicating the formation of lead sulphide is noted, then the presence of sulphur in the organic compound is confirmed.
\(\mathrm{Na}_{2} \mathrm{~S}+\left(\mathrm{CH}_{3} \mathrm{COO}\right)_{2} \mathrm{~Pb} \rightarrow \mathrm{PbS}+2 \mathrm{CH}_{3} \mathrm{COONa}\)
Detection of Phosphorus
The presence of phosphorus in the organic compound can be detected by fusing the organic compound with sodium peroxide, an oxidising agent. Phosphorus present in the organic compound gets oxidised to sodium phosphate.
\(5 \mathrm{Na}_{2} \mathrm{O}_{2}+2 \mathrm{P} \Delta \rightarrow 2 \mathrm{Na}_{3} \mathrm{PO}_{4}+2 \mathrm{Na}_{2} \mathrm{O}\)
The fused mass is extracted with water and the aqueous solution is then boiled with concentrated nitric acid. To this, ammonium molybdate is added. A yellow precipitate or colour (because of the formation of ammonium phosphomolybdate) shows the presence of phosphorus.
Detection of Oxygen
While there are no direct tests to detect the presence of oxygen in an organic compound, the below tests can indirectly confirm its presence.
a. Presence of functional groups or tests to confirm the presence of functional groups such as \(-\mathrm{OH},-\mathrm{COOH},-\mathrm{NO}_2\) etc., indicates the presence of oxygen indirectly.
b. When the sum percentages of elements in the organic compound, determined through quantitative analysis, does not come out to be \(100 \%\), the differential sum indicates the presence of oxygen, and its percentage can also be determined.
Summary of Qualitative Analysis of Organic Compounds: Identification and Characterization
Qualitative analysis of organic compounds gives a detailed indication of the elements present in an organic compound. It is non-quantifiable. When an unknown organic compound is given, it is essential to identify the elements present in it, before detecting the quantities of each element. The identification is done through individual detection tests available for carbon and hydrogen, nitrogen, sulphur, phosphorus and halogens, and oxygen. While these elements have individual tests to confirm their presence in an organic compound, most of them can be detected by preparing the Sodium fusion extract or the Lassaigne’s extract using sodium metal. For oxygen, there are no direct tests available. However, its presence can be detected indirectly using the methods available for predicting functional groups containing oxygen.
FAQs on Qualitative Analysis of Organic Compounds: Identification and Characterization
Q.1. What is qualitative analysis for organic compounds and why it is important?
Ans: The qualitative analysis of organic compound is a non-quantifiable analysis of organic compounds. It is important because it gives the detailed record of the elements present in the organic compounds.
Q.2. Which steps are used in the qualitative analysis of organic compounds?
Ans: Qualitative analysis uses different method of detection or tests for different elements such as oxygen, nitrogen, carbon and hydrogen, sulphur, phosphorus, and halogens. Each of these tests come with different steps in their own regard and need to be carried out individually.
Q.3. Which method is used for detection of Nitrogen in an organic compound?
Ans: Detection of nitrogen is done using three different tests: Dry heating test, Soda lime test, and Lassaigne’s test.
Q.4. How can we detect the presence of sulphur in an organic compound?
Ans: Detection of sulphur can be done using Lassaigne’s extract and reacting it with either sodium nitroprusside to get violet colouration or by using lead acetate and acetic acid to get a yellow precipitate or colouration.
Q.5. What are the types of qualitative analysis?
Ans: Qualitative analysis helps in detection of individual elements in an organic compound. Each element has a set of individual tests which are performed to confirm the presence of that element in the organic compound under investigation.
Learn About Chromatographic Techniques Here
We hope this detailed article on Qualitative Analysis of Organic Compounds is helpful to you. If you have any queries, drop a comment below, and we will get back to you.